How many lemons will it take to power a car?
Lemons aren’t the sweetest fruit, but they certainly carry a fair amount of electricity. Now imagine an electric car, specifically the Tesla Model S. This battery contains about 232Ah and 22.8V. A lemon averages at about 1mA and 0.9V, so powering a car with it would be an insane idea, yet it is still completely possible, if you have the time, that is.
Parallel vs. Series
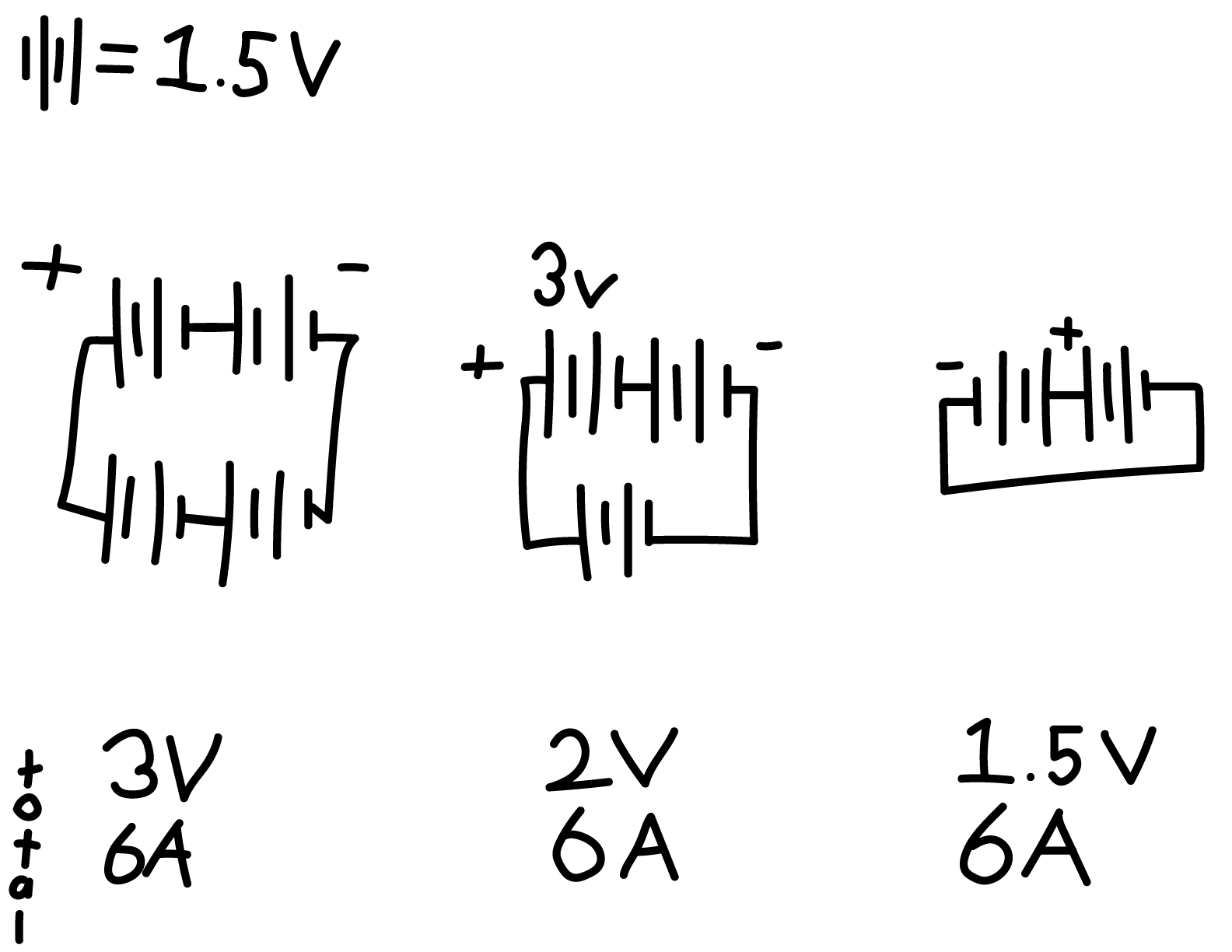
When connecting batteries, a few ways to increase the power of the flow is to connect them together, and there are 2 different methods in doing so. Connecting in parallel combines the ampere-hours, (the measure of battery capacity,) by connecting the positive with the positive and negative with the negative. In parallel, your two AA batteries will last twice as long as one, while keeping the same 1.5 volts. On the other hand, connecting in series sums up the voltage of all batteries. Instead of matching the positive to the positive, you match the positive with the negative. Now, your two AA batteries will only last as long as one, but deliver 3 volts in return. You can connect multiple pairs of batteries in series together with a parallel connection and have both a higher voltage and a higher capacity. Which is what we will be doing to boost 1mA and 0.9V to 232Ah and 22.8V.
From 0.001A to 232Ah
The easiest way to find this information is to run a simple calculation. Theoretically, if every single lemon had a current of 1mA, it would take… 232,000 lemons to get to 232A, and the voltage is still 0.9V as they would be connected in parallel. Which, compared to a rough estimation for how many lemons someone would consume in their lifetime if they drank 5 glasses a day, every day, with each glass containing 2 lemons, and lived to 79 years old, the average life expectancy in the US, that would add up to about 304,150 lemons including leap years. 232,000 is only 72,150 lemons away. However, lemon batteries can last as long as the electrodes and electrolytes in the lemons do.
⌈(e(g)•(365+⌈a/4⌉))•a⌉
This is the setup for the lemons consumed problem, with e being the amount of lemons in a glass, g being the amount of glasses, and a being the age of the person.
When inquiring about this to a mutual about the processes of estimation used to identify these results, it was a very similar process for both problems. Despite solving for a different problem with the amperage, (they went with the amperage needed to power up smaller hybrid type car compared to actually running an electric car,) the same method was used, which was just multiplying by 1000. My explanation for this is very simple, as 1 milliamp is ¹⁄₁₀₀₀ of an amp, so obviously this doesn’t require any more calculations than just multiplying by 1000.
Now, as mentioned before, the voltage is still ~0.9V, meaning that we will need a lot more lemons, and when I say a lot, I really mean a lot. Like a parking lot. And we aren’t just trying to start a car, we are trying to power an electric car, enough to the point where you could probably drive around. So this isn’t a matter of starting a gasoline/hybrid car, this is driving a car with presumably millions of lemons. So how many lemons will we really need?
From 0.9V to 22.8V
As mentioned in Parallel vs. Series, connecting batteries in series, - to +, makes the voltage output of your batteries stronger. But to connect in parallel and series, you need to have an equal amount of capacity. Meaning that you can’t just connect a few more lemons in series and say violà, you need to actually have equivalent currents or the combined power will drop by a noticeable amount. To the left, there is an example of the results when running tests using a circuit simulator.
So, now, continuing on the theoretical that each lemon has the same current and voltage, we can just find how many 0.9V lemons it takes to make 22.8V, round up (better to have a bit of a higher voltage or use a regulator compared to undervolting,) and then multiply the amount by 232,000. Which leaves us with…
6,032,000 lemons
six million, thirty-two thousand
~2 weeks of power
if you can keep the lemons fresh
30,401,280 grams of citric acid
thirty million, four hundred one thousand, two hundred eighty